
Insights+: The US FDA New Drug Approvals in June 2024
Shots:
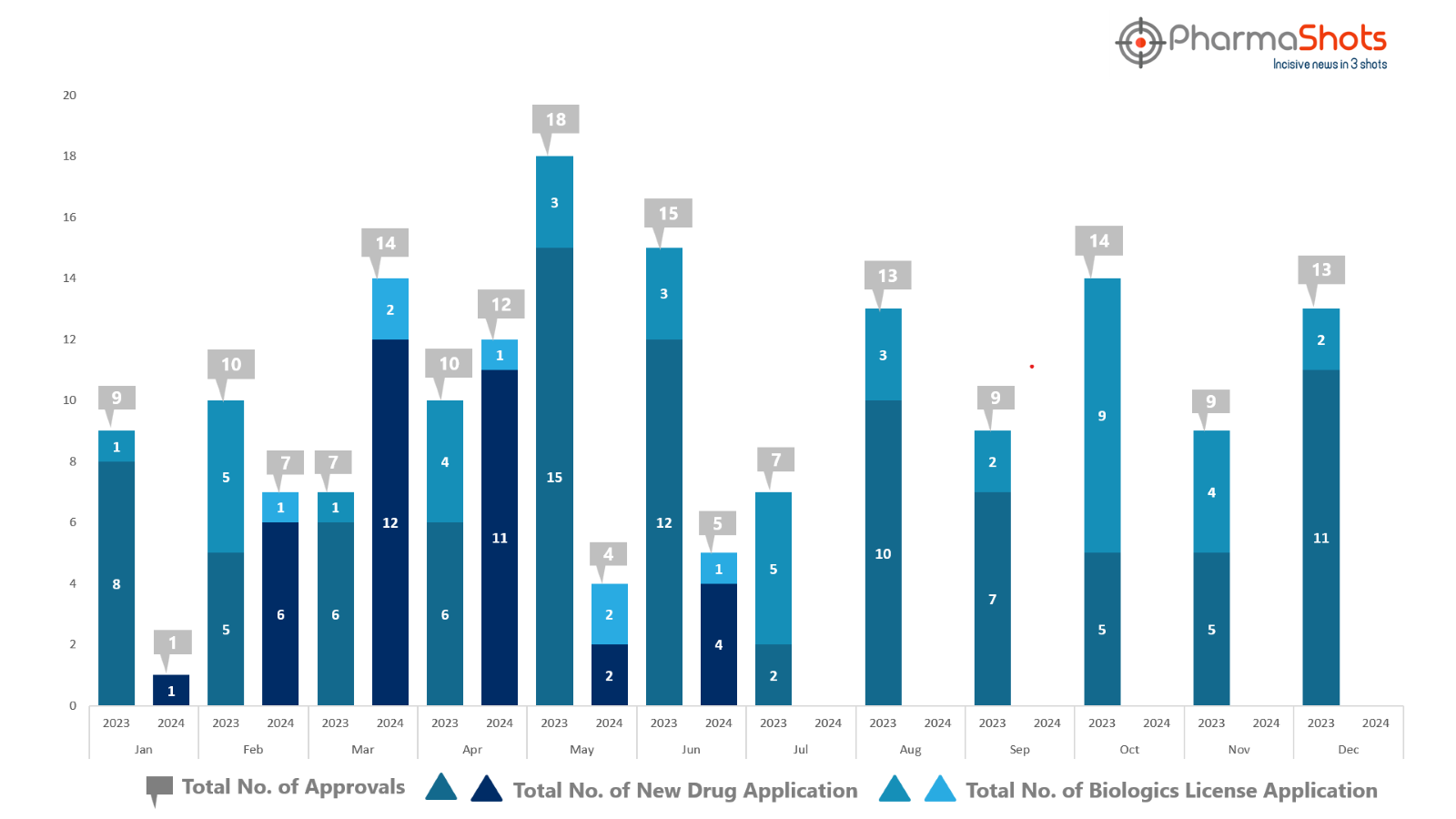
- PharmaShots has compiled a list of US FDA-approved drugs in the month of June 2024
- The US FDA approved a total of 5 new drugs including 4 new molecular entities and 1 biologic leading to the treatments for patients and advances in the healthcare industry
- The major highlighted drug was Ipsen’s Iqirvo for the treatment of Primary Biliary Cholangitis
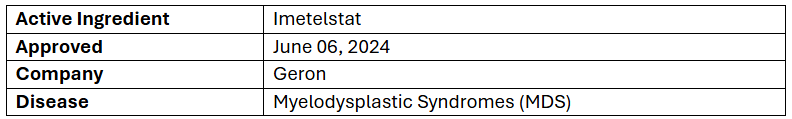
Shots:
- The US FDA has approved Rytelo to treat low- to intermediate-risk MDS with transfusion-dependent (TD) anemia adults who did not respond to, lost response, or are not eligible for erythropoiesis-stimulating agents (ESA)
- The approval was supported by the P-III (IMerge) study of Rytelo vs PBO, depicting a 39.8% vs 15.0% RBC-TI rate for 8wks. and 28.0% vs 3.3% for 24wks. RBC-TI was durable, with median durations of ~1yr. for 8wk. responders & 1.5yrs. for 24wk. responders
- The exploratory analysis showed 3.6 g/dL vs 0.8 g/dL median increase in hemoglobin among patients attaining ≥8wk. RBC-TI with meaningful efficacy in MDS subgroups, regardless of ring sideroblast status, baseline transfusion burden & IPSS risk category
Shots:
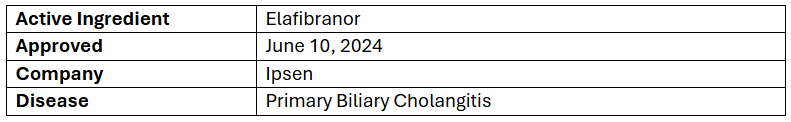
- The US FDA has granted accelerated approval to Iqirvo (80mg tablets) in addition to ursodeoxycholic acid (UDCA) to treat PBC adults having inadequate response to UDCA or as monotx. in UDCA intolerant patients. Full approval depends on (ELFIDENCE) confirmatory trial
- The approval was based on a P-III (ELATIVE) study assessing the safety & efficacy of Iqirvo (80mg, QD) + UDCA (n=108) vs PBO + UDCA (n=53) to treat PBC. Results, published in the NEJM, showed BCR in 51% vs 4% of patients with a 47% treatment benefit & ALP normalization at wk.52 in 15% vs 0% of them, sustained through wk.52 & evident by wk.4
- In addition, the company has submitted Iqirvo for PBC to the EMA & MHRA with the decisions expected in H2’24
Shots:
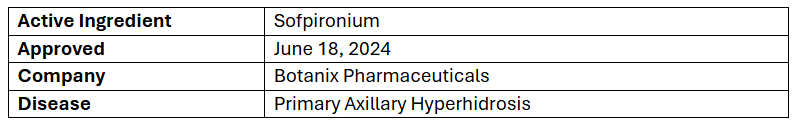
- The US FDA has granted approval to Sofdra gel (12.45%) for the treatment of adults & children (≥9yrs.) with primary axillary hyperhidrosis
- The approval was based on the two pivotal P-III (CARDIGAN) trials assessing the efficacy & safety of Sofdra vs vehicle in primary axillary hyperhidrosis patients (n=701). Trials reached its 1EPs & 2EPs showing improved GSP & HDSM-AX7 score
- The patient experience program for the same is anticipated in Q3’24 with the introduction of Sofdra in Q4’24
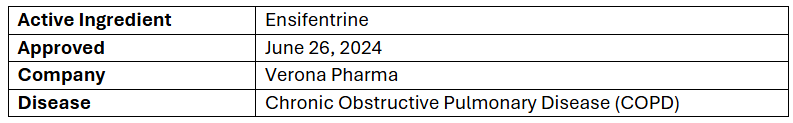
Shots:
- The US FDA has approved Ohtuvayre as a maintenance therapy to treat COPD patients, with its launch anticipated in Q3’24
- The approval was based on the P-III (ENHANCE-1 & ENHANCE-2) studies analyzing Ohtuvayre monotx. or in addition with other maintenance therapies. Trials reached their 1EPs showing improved lung function; results were published in the American Journal of Respiratory and Critical Care Medicine
- Ohtuvayre is a dual PDE3 & PDE4 inhibitor that possesses both bronchodilator & non-steroidal anti-inflammatory activities. It is administered using a standard jet nebulizer into the lungs
Note: According to the FDA's June 2024 approval list, the following drug was also approved; however, no PR was available:
-
PiaSky
Related Post: Insights+: The US FDA New Drug Approvals in May 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














